Enhanced TDS
Identification & Functionality
- Chemical Family
- Chemical Name
- Base Chemicals Functions
- Technologies
- Product Families
- Chemical Structure
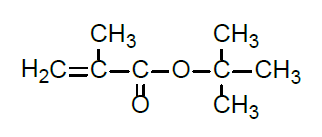
Features & Benefits
- Benefits
tert-Butyl Methacrylate (TBMA) is a monofunctional monomer with a characteristic high reactivity of methacrylates and a bulky hydrophobic moiety. tert-Butyl Methacrylate (TBMA) can be used to impart the following properties to polymers:
- Chemical resistance
- Hydrophobicity
- Hardness
- Scratch resistance
- Adhesion
- Heat resistance
- High solids
- Weatherability
Applications & Uses
- Markets
- Applications
- Applications
- tert-Butyl Methacrylate (TBMA) forms homopolymers and copolymers. Copolymers of tert-Butyl Methacrylate (TBMA) can be prepared with acrylic acid and its salts, amides and esters, and with methacrylates, acrylonitrile, maleic acid esters, vinyl acetate, vinyl chloride, vinylidene chloride, styrene, butadiene, unsaturated polyesters and drying oils, etc.
- tert-Butyl Methacrylate (TBMA) is also a very useful feedstock for chemical syntheses, because it readily undergoes addition reactions with a wide variety of organic and inorganic compounds.
Properties
- Physical Form
Packaging & Availability
- Country Availability
- Packaging Type
- Packaging Information
- 850KG Composite IBC
Storage & Handling
- Storage Information
- In order to prevent polymerization, tert-Butyl Methacrylate (TBMA) must always be stored under air, and never under inert gasses. The presence of oxygen is required for the stabilizer to function effectively. It has to contain a stabilizer and the storage temperature must not exceed 35°C. Under these conditions, a storage stability of one year can be expected upon delivery. In order to minimize the likelihood of overstorage, the storage procedure should strictly follow the “first-in-first-out” principle. For extended storage periods over 4 weeks it is advisable to replenish the dissolved oxygen content.
- The preferred construction material for tanks and pipes is stainless steel. Carbon steel is also acceptable, although the formation of rust may be a problem with product quality (color). Iron(III)-ions have been shown to be a weak polymerization initiator.
- If carbon steel is to be used, special procedures should be used to prepare the tank for use. Storage tanks, pumps and pipes should be earthed.
