Enhanced TDS
Identification & Functionality
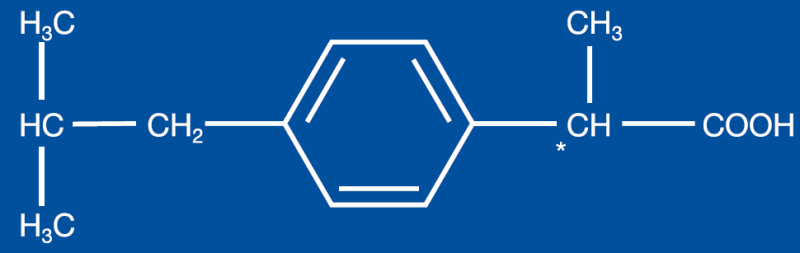
- Chemical Name
- Country of Origin
- Ingredient Origin
- Pharma & Nutraceuticals Functions
- Technologies
- Product Families
- Structural Formula
Features & Benefits
- Benefit Claims (Health)
- Labeling Claims
- Key Benefits
- Ibuprofen DC 85 W is able to be compressed into tablets with minimum amount of lubricant
- Excellent tablet engraving
- Maximizes production speed while minimizing stickiness
- Allows for smaller tablet sizes compared to those with pure Ibuprofen which are easier to swallow
Applications & Uses
- Markets
- Applications
- Dosage Form
- Manufacturing Technology
- Recommended Applications
- Due to its analgesic, antipyretic and anti-inflammatory actions, it is used in the treatment of inflammatory conditions such as rheumatoid arthritis, osteoarthritis, mild to moderate pain, dysmenorrhea, headache, and fever.
- The common active ingredient dosage in tablets is 200, 400, 600 and 800 mg. The OTC dosage forms are mainly the 200 and 400 mg forms (except for the United States and some other countries, where the 200 mg form is the only OTC form). Other common dosage forms are capsules, syrups, suspensions, suppositories, and topical dosage forms like creams and gels.
- Processing of Ibuprofen
- Oral film-coated tablets showing rapid disintegration and fast release of the active substance. The common strengths are 200, 400, 600 and 800 mg. There are also slow release formulations containing 800 mg of Ibuprofen.
- Oral suspensions which are used mainly for patients who have difficulties swallowing tablets and for pediatric patients.
- Creams and gels for topical application, generally used for treating rheumatic disorders or sports injuries.
- Recommendation for Direct Compression
- Today the manufacturing of ibuprofen tablets is often done by direct compression. Using this method, the expensive and time-consuming wet granulation method can be avoided. But in general, ibuprofen has the disadvantage of sticking on the tablet tools so that the process must be interrupted often.
- Therefore, direct compression formulations with a high content of ibuprofen per tablet are often avoided. Mostly tablets with an ibuprofen content of maximum 60% are compressed.
- BASF offers a formulated ibuprofen product ideal for direct compression: Ibuprofen DC 85 W. The direct compression (DC) grade ensures that tablet sticking is minimized and allows for excellent tablet engraving.
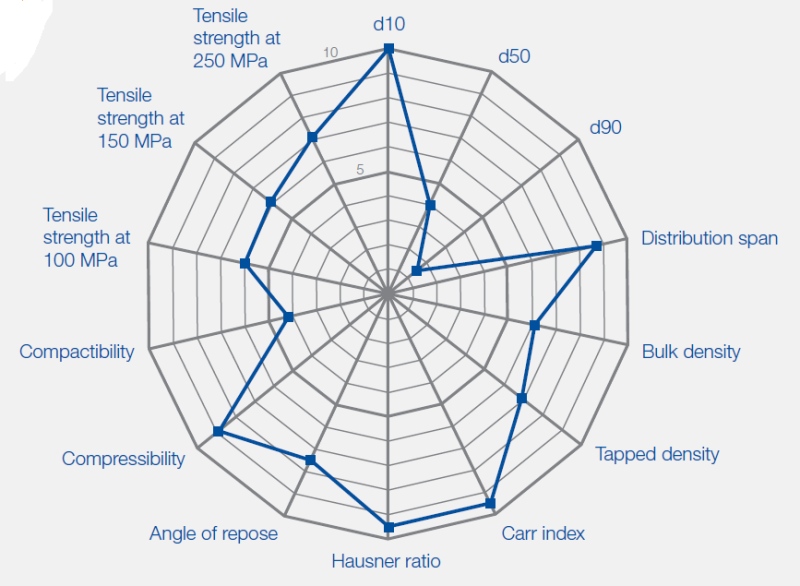
- Furthermore, Ibuprofen DC 85 W has a lower angle of repose compared to standard grades, resulting in improved flowability.
Properties
- Physical Form
- Appearance
- White granules, free-flowing, homogeneous material
- Typical Properties
Value Units Test Method / Conditions Angle of Repose 33 degree - Tapped Density approx. 0.64 g/ml - Bulk Density approx. 0.55 g/ml - - Composition
Value Units Test Method / Conditions Croscarmellose Sodium, Microcrystalline Cellulose, Colloidal Silicon Dioxide 15 % - Ibuprofen 85 % - - Sieve Test
Value Units Test Method / Conditions Particle Size (Retained on 0.3mm Sieve) min. 45 % - Particle Size (Retained on 0.85mm Sieve) min. 15 % -
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- Grade
- Regulatory Information
The ibuprofen used to manufacture Ibuprofen DC 85 W meets the current Ph. Eur., USP, JP and IP monographs.
Technical Details & Test Data
- SEM Photograph

- Formulation Guidance
- Direct compression is possible
- Dry granulation (roller compaction) is possible
- Wet granulation (fluid-bed or high-shear granulation) is possible
Packaging & Availability
- Country Availability
- Packaging Type
- Packaging Information
- 50KG Fiber drums
Storage & Handling
- Storage Information & Retest Period
- Store tightly closed. Carefully reseal the container after opening
- Retest Period : 36 months
